Over 45,000 cases of mucormycosis reported in country
A total of 45,432 cases of mucormycosis have been reported by states and UTs till July 15 of which 21,085 affected people are receiving treatment and 4,252 have died, the Rajya Sabha was informed on Tuesday.
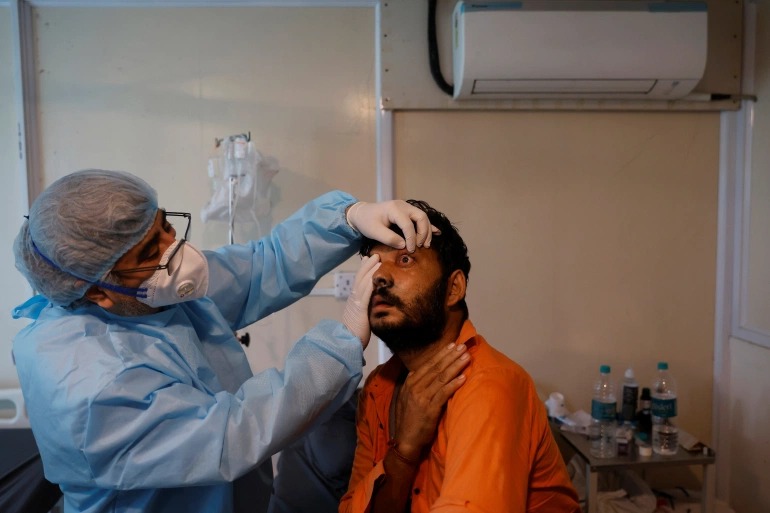
New Delhi: A total of 45,432 cases of mucormycosis have been reported by states and UTs till July 15 of which 21,085 affected people are receiving treatment and 4,252 have died, the Rajya Sabha was informed on Tuesday.
A large majority of mucormycosis patients (84.4 per cent) had reported a history of COVID-19, Union Health Minister Mansukh Mandaviya said in a written reply. Most common presentations of mucormycosis include Rhinocerebral (77.6 per cent), Cutaneous (4.3 per cent) and Pulmonary (3.0 per cent).
In the wake of second wave of COVID-19, an increased number of mucormycosis cases were reported and the Ministry of Health after thorough gap analysis and consultations took a number of steps to address the problem.
Mucormycosis and other fungal infections are most commonly seen as an opportunistic infection.They are found in those with low underlying immunity either due to a diabetes, cancers particularly hematological malignancies, etc. or as a side effect of prolonged/irrational use of certain drugs like steroids, immunosuppressive drugs for management of other disorders including COVID-19, the minister explained.
Mandaviya said although it is not a new disease, its true incidence during the beginning of second wave was unknown as it was not a notifiable disease.
The Ministry of Health in May requested states to declare Mucormycosis a notifiable disease under the Epidemic Disease Act to get an objective assessment of the disease.
Taking cognizance of association between elevated blood sugar levels (whether in patients with pre-existing diabetes mellitus, or hyperglycemia due to steroid therapy), an updated Clinical Guidance on Diagnosis and Management of Diabetes at COVID-19 Patient Management facility was issued by the ministry on June 1.
Also Read |
AIADMK MP V Maitreyan breaks down during farewell speech in Rajya Sabha
After expert deliberations in the National Task Force on COVID-19, a detailed advisory on treatment and management of COVID-19 associated Mucormycosis (CAM) was formalized and disseminated on June 7.
Amphotericin-B has been prescribed and it is available in two formulations, Liposomal Amphotericin B and Amphotericin B Deoxycholate, which are similar in efficacy.
However, the latter needs to be used with greater caution with respect to its effects on kidney function test and electrolyte imbalance, the minister explained.
The second drug of choice is Posaconazole which may be used in cases with compromised renal function, drug reaction to Amphotericin B, electrolyte imbalance or in non-availability of Amphotericin B.
A checklist for managing mucormycosis and other fungal infections was also circulated to all states and UTs on June 11.
The clinical management guidelines for managing COVID-19 cases by the ministry advocates rational use of steroids for managing moderate to severe cases of COVID-19 under medical supervision.
For the reporting of cases and deaths due to mucormycosis, the ministry has requested the states and UTs to submit the details on the COVID-19 India portal.
Also Read |
Economic Survey: India to become a five trillion dollar economy by 2025
States were asked to update the data on the portal which has helped the ministry to analyse and keep a track of evolving nature of problem.
In response to a question on whether the government has taken cognisance of shortage in supply of Amphotericin-B injections used for the treatment of mucormycosis, Mandaviya said since early May the details of production, stock, supplies made and purchase orders were obtained from manufacturers and their cooperation was sought to overcome the gap between supply and demand.
Both first and second line drugs used for managing mucormycosis cases are currently amply available in Indian markets, he said.
The Department of Pharmaceuticals (DoP), Central Drugs Standard Control Organisation (CDSCO) & National Pharmaceutical Pricing Authority (NPPA) have been jointly monitoring the production and availability of critical Covid drugs including Amphotericin-B injections, he said in the reply. The Centre has taken various measures to improve the availability of Amphotericin B (liposomal) through a multipronged approach of augmenting production, and import and ensure equitable distribution to states and UTs. It is also engaging with manufacturers to resolve issues related to raw materials.
The Department of Pharmaceuticals and the Indian Embassy in the US are working continuously with Mylan Labs for increasing import and ensuring early delivery from M/s Gilead Inc. USA.(PTI)
 Dynamite News
Dynamite News 